UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of February 2022
Commission File Number: 001-40212
Connect Biopharma Holdings Limited
(Translation of registrant’s name into English)
Science and Technology Park
East R&D Building, 3rd Floor
6 Beijing West Road, Taicang
Jiangsu Province, China 215400
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
On February 16, 2022, Connect Biopharma Holdings Limited (the “Company”) presented certain company highlights and financial information at the SVB Leerink 11th Annual Global Healthcare Conference. A copy of the materials presented are attached hereto as Exhibit 99.1.
The information set forth in the attached presentation shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or under the Securities Exchange Act of 1934, whether made before or after the date hereof, except as expressly provided by specific reference in such a filing.
The furnishing of the attached presentation is not an admission as to the materiality of any information therein. The information contained in the corporate presentation is summary information that is intended to be considered in the context of more complete information included in the Company’s filings with the Securities and Exchange Commission (the “SEC”) and other public announcements that the Company has made and may make, by press release or otherwise, from time to time. The Company undertakes no duty or obligation to update or revise the information contained in this report, although it may do so from time to time as its management believes is appropriate. Any such updating may be made through the filing or furnishing of other reports or documents with the SEC, through press releases, by updating its website or through other public disclosures.
Exhibit Index
| Exhibit No. |
Description | |
| Exhibit 99.1 | SVB Leerink 11th Annual Global Healthcare Conference Presentation | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Dated: February 16, 2022 | CONNECT BIOPHARMA HOLDINGS LIMITED | |||||||
| By | /s/ Steven Chan | |||||||
| Name: | Steven Chan | |||||||
| Title: | Chief Financial Officer | |||||||

Leerink Conference Presentation – February 2022 NASDAQ: CNTB DEVELOPING NEXT-GENERATION THERAPEUTICS FOR T CELL DRIVEN INFLAMMATORY DISEASES Exhibit 99.1

Forward-Looking Statements This presentation regarding Connect Biopharma Holdings Limited ("Connect," "we," "us" or "our") has been prepared solely for informational purposes. Certain information contained in this presentation relates to, or is based on, studies, publications, surveys and other data obtained from third-party sources and Connect’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial condition, results of operations, business strategy and plans, prospective products, product approvals, anticipated milestones, expected data readouts, research and development plans and costs, timing and likelihood of success, objectives of management for future operations, future results of anticipated product development efforts and adequacy of existing cash to fund operations, as well as statements regarding industry trends, are forward-looking statements. Forward-looking statements can be identified by words such as: “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” or the negative of these terms or other similar expressions. The forward-looking statements in this presentation are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are inherently subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control, including, among other things: the ability of our clinical trials to demonstrate safety and efficacy of our product candidates, and other positive results; our ability to obtain and maintain regulatory approval of our product candidates; existing regulations and regulatory developments in the United States, the PRC, Europe and other jurisdictions; uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations; risks associated with the COVID-19 outbreak, which has and may continue to materially and adversely impact our business, preclinical studies and clinical trials; our plans and ability to obtain, maintain, protect and enforce our intellectual property rights and our proprietary technologies, including extensions of existing patent terms where available; our continued reliance on third parties to conduct additional clinical trials of our product candidates, and for the manufacture of our product candidates for preclinical studies and clinical trials; and the degree of market acceptance of our product candidates by physicians, patients, healthcare payors and others in the medical community. These risks are not exhaustive. The inclusion of forward-looking statements should not be regarded as a representation by Connect that any of its expectations, projections or plans will be achieved. Actual results may differ from those expectations, projections or plans due to the risks and uncertainties inherent in Connect’s business and other risks described in Connect’s filings with the SEC. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this presentation, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.

Validated, T cell-modulating discovery approach designed to enhance and speed up the identification of potentially highly differentiated immune modulators CBP-201:- Interleukin-4-receptor alpha (IL4Ra) blocker CBP-307:- Sphingosine 1-phosphate-1 (S1P1) modulator CBP-174:- Peripherally acting Histamine-3 Receptor (H3R) antagonist Seasoned industry leaders with significant experience in immunology drug discovery and development; building a global internal and external team to drive development in USA, Europe, China and Rest of World ~$268 million USD in cash and cash equivalents at year-end 2021 (unaudited)* Company Highlights Prolific Discovery Engine Clinical Stage with Deep Pipeline Global Outlook Strong Cash Position Targeting inflammatory diseases (Dermatology, Gastroenterology, Respiratory) with high unmet need Ambition to develop molecules with “First-in-class” or “Best-in-class” potential Large Opportunity Anticipated 2x clinical trial data read outs by end 1st Half 2022, and a total 4x clinical trial data read outs by end 1ST Half 2023, across 4x diseases Multiple Catalysts * This is management’s preliminary estimate and subject to audit by the Company’s independent registered public accounting firm.
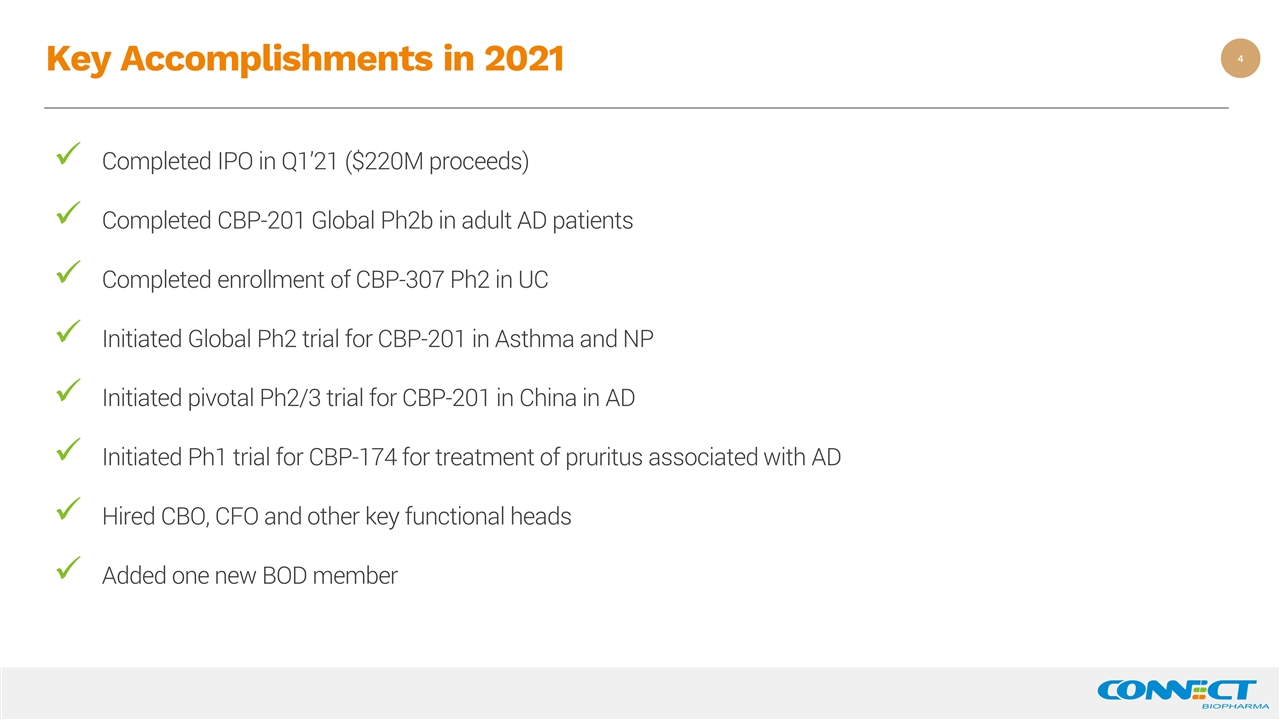
Key Accomplishments in 2021 Completed IPO in Q1’21 ($220M proceeds) Completed CBP-201 Global Ph2b in adult AD patients Completed enrollment of CBP-307 Ph2 in UC Initiated Global Ph2 trial for CBP-201 in Asthma and NP Initiated pivotal Ph2/3 trial for CBP-201 in China in AD Initiated Ph1 trial for CBP-174 for treatment of pruritus associated with AD Hired CBO, CFO and other key functional heads Added one new BOD member

A pipeline of potentially highly differentiated therapies Connect Biopharma has Global Development & Commercialization Rights to all Product Candidates INDICATION PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NEXT ANTICIPATED MILESTONE CBP-201 Antibody targeting IL-4Rα cytokine receptor (Th2 cell modulator) Atopic Dermatitis (AD) Initiate Global Ph3 in 2H’2022 Asthma Report Ph2 top-line in 1H’2023 Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) Complete full enrolment in 2H’2022 CBP-307 Small molecule targeting S1P1 (Th1 cell modulator) Ulcerative Colitis (UC) Report Ph2 top-line in 1H’2022 Crohn’s Disease (CD) * Determine upon completion of Ph2 UC trial CBP-174 Peripherally restricted H3 receptor antagonist Pruritus associated with AD Report Ph1 top-line data in 1H’2022 * Phase 2 trial ended early due to COVID-19-related enrolment challenges. Future clinical development plans to be determined upon completion of Ph2 UC trial.

NASDAQ: CNTB
